Cis-trans stereo
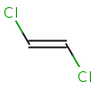
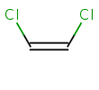
In general, single bonds are rotatable, but double bonds are not. If the substituents on each side of the double bond are different, then two diastereomers of the molecule can be distinguished based on the orientation of the ligands. Two substituents located on the same side of the double bond are referred to as cis isomer, otherwise, if the two substituents are located on the opposite side it is referred to as trans isomer.
|
|
|
|
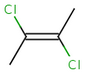
trans-1,2-dichloroethene |
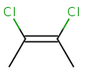
cis-1,2-dichloroethene |
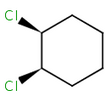
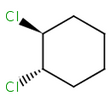
Alicyclic compounds can also display cis-trans isomerism. In this case a single bond becomes non rotatable due to constrain of a cycle. However, in these cases we use tetrahedral stereochemistry.
|
|
|
|
(1R,2S)-1,2-dichlorocyclohexane |
(1S,2S)-1,2- |
E/Z notation
The cis/trans system for naming isomers is not effective if more than two different substituents are attached to the double bond. In this case, following the Cahn-Ingold-Prelog priority rules, a priority is assigned to each substituent on a double bond. If the two groups of higher priority are on opposite sides of the double bond (trans arrangement), then the E configuration is assigned to the bond. If the two groups of higher priority are on the same side of the double bond (cis arrangement), than the Z configuration is assigned to it.
|
|
|
|
2E-2,3-dichlorobut-2-ene |
2Z-2,3-dichlorobut-2-ene |
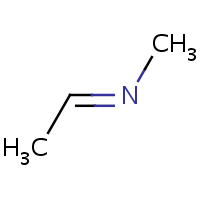
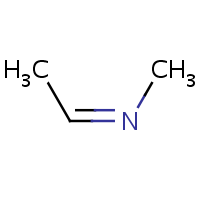
E/Z stereochemistry of the nitrogen atom is also supported:
|
|
|
|
(E)-ethylidene(methyl)amine |
(Z)-ethylidene(methyl)amine |