Tautomerization and tautomers
This page explains the concepts behind our tautomer generation process:
Introduction
Tautomers are structural isomers of organic compounds that are in dynamic equilibrium due to the migration of a proton. The isomerization reaction by which tautomers are interconverted is called tautomerization.
In the following picture X, Y and Z atoms can be C, H, O or S atom, and H becomes an electrophile center during isomerization. When the electrophile center is a H+, the isomerization is also known as prototropy.

Fig. 1 Tautomerization (isomerization) example
In solutions - in which tautomerization is possible - a chemical equilibrium of the isomers will be reached while the reaction results in the formal migration of a hydrogen atom or proton accompanied by a switch of a single bond and an adjacent double bond. Usually the catalysts of these reactions are acids or bases.
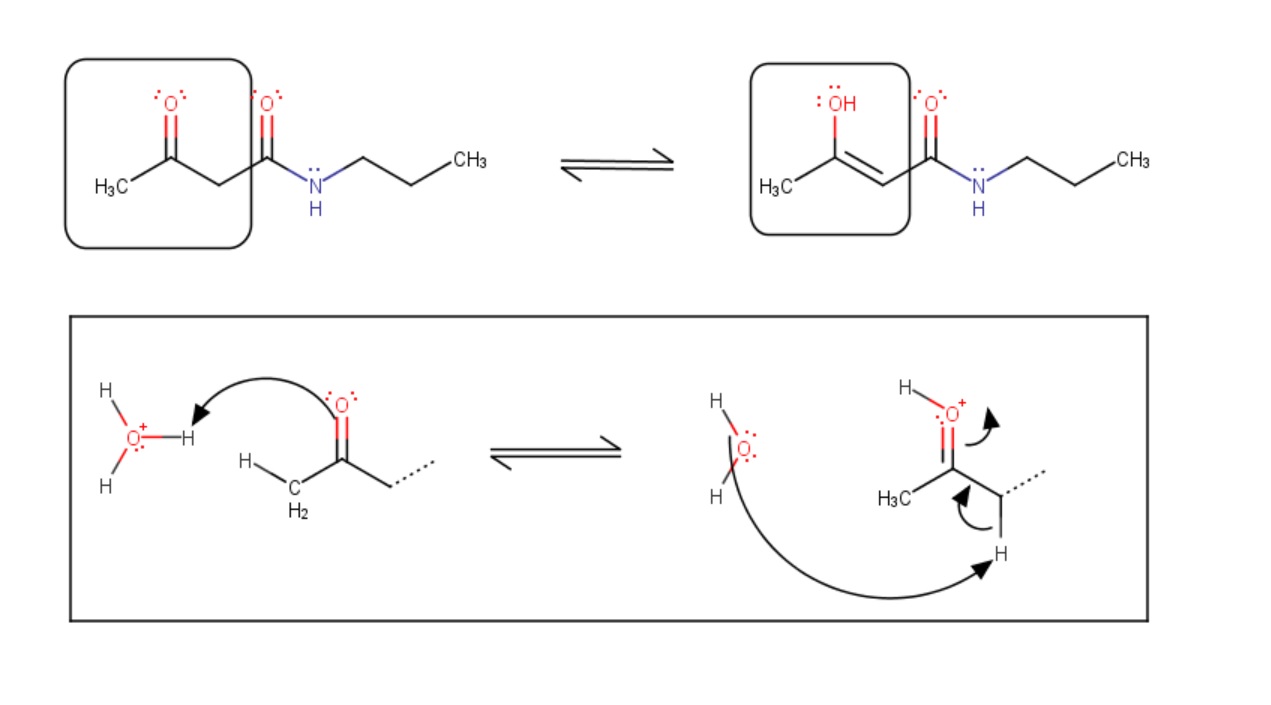
Fig. 2 Chemical equilibrium between tautomers and the schematic mechanism of the tautomerization
Tautomer forms in the tautomerization model
Different tautomers forms of a chemical compound can be generated with the tautomer generator. It first identifies all possible proton donors and acceptors in a molecule and finds tautomerization paths between them. Then depending on the tautomer form one wants to calculate, the tautomerization algorithm can
-
combine the tautomer paths into tautomer regions (resulting in a generic tautomer representation)
-
combinatorially generates all possible tautomer forms (resulting in the set of all tautomers)
-
filter and rank the enumerated forms (resulting in a major tautomer form or dominant tautomer distribution);
-
it can provide a (unique) canonical tautomer form with canonicalization of all forms
-
normal forms by applying normalization
Canonicalization applies systematic rearrangement of single and double bonds in the available tautomer regions, resulting in a unique tautomer form that can theoretically represent the whole tautomer set of the input structure.
With the Normal
tautomer
generation option the tautomer forms are generated according to empirical rules. Normal tautomer generation narrows down the possible tautomerization paths and leads to chemically more feasible products. You can find detailed description of the different tautomer generation options here.
The normal tautomer generation mode corresponds to the old rational tautomer generation mode.
Comparison of the generated tautomer forms - example
The example below illustrates the different generated tautomer forms for the example molecule.
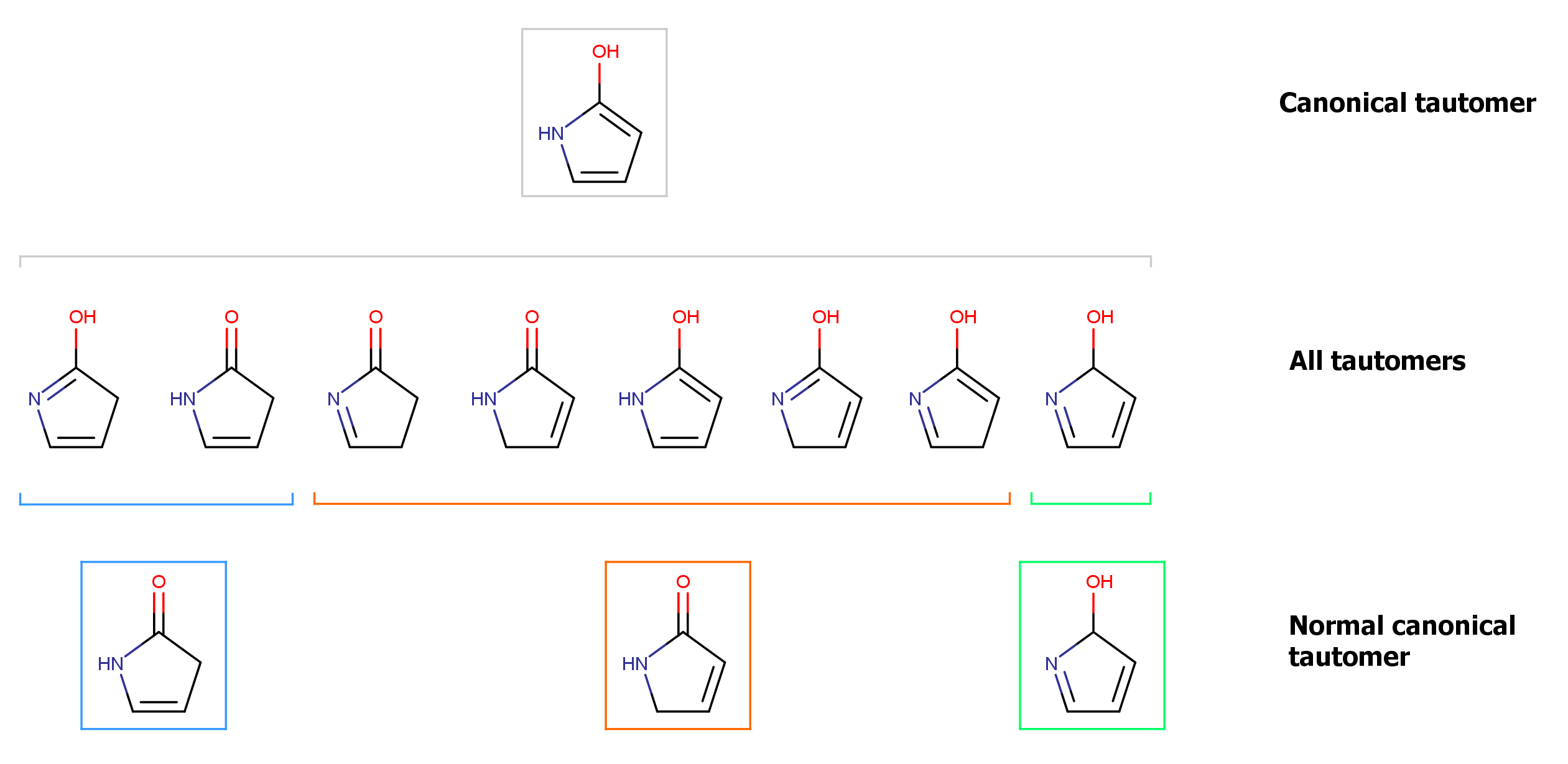
Fig. 3 Different tautomer forms generated for the input molecule
This example illustrates how the applied rules for tautomer generation narrows down the generated number of tautomers. The canonical form represents a unique form that can be regarded as a "theoretical" representation of the whole tautomer set.
Normal canonical forms are representations of subsets of the whole tautomer set. Such a subset is narrowed down into its normal canonical form by introducing chemical rules into the generation process. (A chemical rule is a consideration whether a tautomer transformation between two forms can happen.) Tautomer forms within a subset can all be converted into each other, and their normal canonical form will be the same, too. However, forms that are elements of two different subsets (e.g. blue and red subset in the example) cannot be transformed into each other according the normal canonical tautomerization rules, and they will have different normal canonical forms, too.