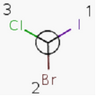
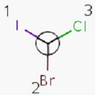
Tetrahedral Stereo
The dimension of a molecule can be interpreted topologically, based on the connections of the consisting atoms, or spatially, based on the Cartesian coordinates of them. In this section the notion of dimension is used in spatial sense.
Molecules with same connectivity but different spatial arrangement are called stereoisomers.
Stereoisomer types:
-
Enantiomers: Molecules that are non-superimposable, complete mirror images of each other.
-
Diastereomers: Stereoisomers that are not enantiomers.
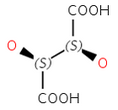
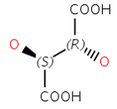
Special cases
-
Non-carbon tetrahedral stereocenters
-
Ammonium and phosphonium salts
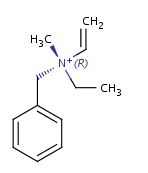
-
Amine oxides and phosphanones
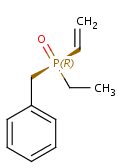
-
Phosphanes
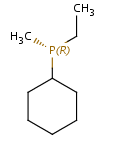
In this case the lone pair of phosphorus atom is considered as the fourth ligand.
-
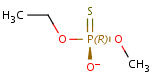
Phosphates and phosphonates
-
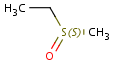
Sulfoxides
-
-
An atom in a ring is a tetrahedral center, if
-
the central atom has 2 different kinds of ligands outside the ring, and
-
the graph invariant of the ring is not the same in the two sides of the central atom, or
-
the graph invariant of the ring is the same in the two sides of the central atom, but
-
the ring contains even numbers of atoms (including the parity central atom), or
-
there is an atom with nonzero parity in the opposite side of the ring:
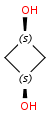
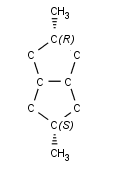
-
-
-
Nitrogen atoms in a ring is tetrahedral stereo center, if
-
they are bridgehead atoms.
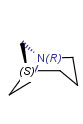
-
-
N is a tetrahedral stereo center in a 3 membered ring,
-
if the graph invariant of the ring is not the same in the two sides of the nitrogen atom.
-
Representation in 0D, 2D and 3D
-
0D: Stereoinformation is defined in 0 dimension by parity.
-
2D: Stereoinformation in 2 dimension is defined by wedge, hatch or wiggly bond types.
-
3D: Stereoinformation in 3 dimension is defined by the coordinates.
Chirality
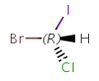
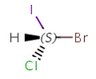
An atom in the molecule around which the ligands are arranged so that interchange of two ligands leads to stereoisomer is called stereocenter orstereogenic center. Chirality appears in stereoisomerism which is due to tetrahedral stereogenic centers. These centers can have point chirality. The ligands of the chiral center are assigned a priority based on the Cahn-Ingold-Prelog priority rules. Each chiral center is then labeled by R or S based on the orientation of the assigned numbers. The center is oriented so that the lowest-priority is pointed away from the viewer. If the priority of the remaining three substituents decreases clockwise, it is labeled R, otherwise, if it decreases counter clockwise, it is S.
|
|
|
|
R-bromo(chloro)iodomethane |
S-bromo(chloro)iodomethane |
Pseudo-asymmetric atoms which are invariant on reflection in a mirror are labeled by r/s descriptors:
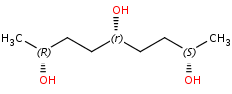
Cahn-Ingold-Prelog priority rules
Explained in Wikipedia: Cahn-Ingold-Prelog priority rules.