Standardizer in a Nutshell
There does not exist a strict convention that defines how to represent chemical structures. Consequently, molecules can be displayed in multiple ways. For example, chemists can draw an aromatic ring in aromatized form or by using alternating single and double bonds, draw hydrogen atoms explicitly or use only implicit hydrogens. In addition, the same molecule can also have different tautomer and mesomer representations.
Thus, even a simple structure like nitrobenzene can be depicted in eight different ways if we consider two distinct forms of the benzene moiety and the nitro functional group and draw hydrogens implicitly or explicitly:
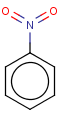
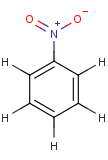
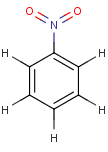
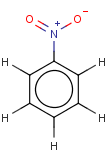
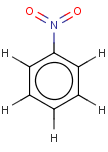
After selecting the desired chemical form of molecules, Standardizer transforms the structures to customized, canonical representations.
For example, the preference can be set to aromatic ring, charged nitro group and implicit hydrogens and all other nitrobenzene representations will be converted to the requested chemical form:
This process eliminates database inconsistencies and produces clean and transparent chemical libraries.
Visit the following page to find out which common chemoinformatics tasks are promoted by Standardizer: