15th Group
Nitrogen (N)
-
Default: NH3
-
Molecules:
-
Neutral atoms
-
Implicit hydrogens are added to have 3 bonds (if nitrogen has at least 1 bond).
More than 3 bonds are forbidden.
If nitrogen has 4 ligands, charge is not set, hence not accepted
Pentavalent N is accepted in traditional form by default, but keep in mind that these forms are not correct. Use the "ylide" from of the N atom which satisfies the correct valence electron count:traditional form
"ylide" form
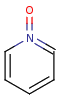
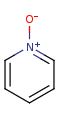
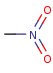
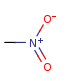
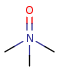
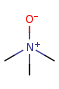
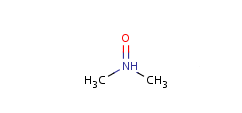
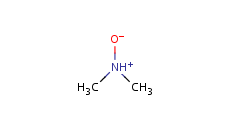
-
It's possible to accept only the "ylide" form using the MoleculeGraph.setValenceCheckOptions() function.
-
-
Charged atoms in a molecule:
-
Positive charge:
-
1+: 4 bonds needed (incluing implicit hydrogens)
-
2+: 3 bonds needed (incluing implicit hydrogens)
-
3+: 2 bonds needed (incluing implicit hydrogens)
-
4+: 1 bonds needed (incluing implicit hydrogens)
-
5+: 0 bonds needed (incluing implicit hydrogens)
-
6+ or more: not allowed
-
-
Negative charge: Every added negative charge decreases by one the number of possible bonds. Maximum charge accepted is -3.
-
-
Radical: Every added radical on these atoms decreases by one the number of bonds. Maximum three added radical is allowed.
-
Aromatic compounds: Nitrogen may form aromatic bonds as well.
-
Phosphorus (P), Arsenic (As), Antimony (Sb), Bismuth (Bi)
-
Default: PH3, AsH3, SbH3, Bi
-
Molecules:
-
Neutral atoms:
-
1 bond: 2 implicit Hydrogens are added.
-
2 bond: 1 implicit Hydrogen is added.
-
3 bond: 0 implicit Hydrogen is added.
-
4 bond: 1 implicit Hydrogen is added.
-
5 bond: 0 implicit Hydrogen is added.
-
More than 5 bonds are forbidden
-
-
Charged atoms in a molecule:
-
Positive charge:
-
1+: 4 bonds are necessary (including implicit Hydrogens).
-
2+: 3 bond is necessary (including implicit Hydrogens).
-
3+: 2 bond is allowed.
-
4+: 1 bond is necessary (including implicit Hydrogens).
-
5+: 0 bond is allowed.
-
6+ or more: not allowed
-
-
Negative charge: Every added negative charge decreases by one the number of possible bonds. Maximum charge accepted is -3.
-
-
Radical: Every added radical on these atoms decreases by one the number of bonds. Maximum three added radicals are allowed.
-
Aromatic compounds: Phosphorus may form aromatic bonds as well.
-