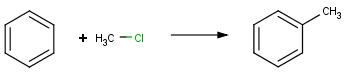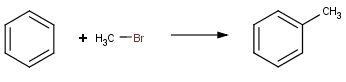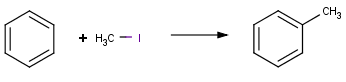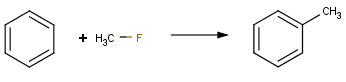Advanced Reaction Scheme Drawing
We can easily define a general transformation of starting compounds in Reactor as described previously, but for being able to perform smart reactions that meet the chemist's expectations, we need to add more elements to the reaction scheme.
Two concepts will be introduced to achieve this:
Narrowing the reactant pool by drawing hydrogens explicitly
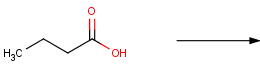
Let's have a look at the reaction scheme of the oxidation reaction of alcohols to carboxylic acids again:

According to this reaction scheme, all reactants that have a hydroxyl group connected to a carbon atom with a single bond will be transformed. Isn't this a too general formulation?
If our reactant set is diverse in functional groups, e.g. carboxylic acids will be transformed as well besides alcohols:
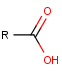
This type of compounds clearly behave differently in chemical reactions than alcohols but satisfy the criterion of having a hydroxyl group connected to a carbon atom with a single bond. Thus, too general representations may cover a bigger chemical space than intended.
We should thus define the environment of the hydroxyl connected carbon in order to exclude carboxylic acids from the reaction definition.
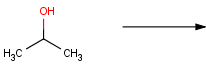
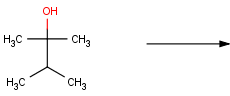
In order to tackle this problem, we should fix the connecting atoms of the hydroxyl carbon. Since oxidation of secondary alcohols terminates at the ketone stage and tertiary alcohols are resistant to oxidation, only primary alcohols are required to satisfy the reaction definition:
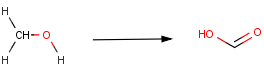
In the new reaction scheme, we defined two additional H atoms connected to the hydroxyl carbon by drawing them explicitly. Accordingly, three valences of the carbon are already fixed, and thus carbonyl carbons (having a double bond to an oxygen) are automatically excluded as well as secondary and tertiary alcohols:
|
|
|
|
|
|
|
|
Note, that this works similarly for the OH group. If the H had been drawn implicitly in the original scheme, a wider definition would have been given for the reactant and e.g. ethers could be included as starting compounds.
Hydrogen atoms should be presented explicitly in the reaction scheme to fix hydrogen connections in the reactant and product molecules.
Introducing variable structural parts by using list atoms
Consider the above reaction scheme but this time we would like to terminate the alcohol oxidation at an intermediate state producing aldehydes. However, ketones, another type of oxocompounds can be produced as well from the oxidation of alcohols if the reactant is a secondary alcohol.
Let's try to give a joint reaction scheme for the oxidation of alcohols to oxocompounds.
As a first attempt, we can simply concentrate on the functional group transformation itself and write the reaction scheme as:

We should then improve this scheme to achieve bigger specificity while including both types of reactions:
|
Reaction |
Example |
|
Primary alcohol oxidation to aldehydes |
|
|
Secondary alcohol oxidation to ketones |
|
The structural difference between primary and secondary acohols is that in secondary alcohols there is an additional linkage to an alkyl group from the C carrying the OH group. Accordingly, we can introduce a variable part of the structure that can either be a C or a H to describe both types of alcohols:
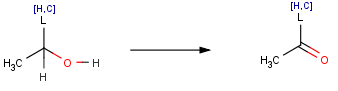
Here the list atom can be either a H or a C as defined in the brackets. Depending on the reactant structure, either an aldehyde or a ketone will be produced in this reaction.
Variable structural parts can be presented as list atoms in the reaction scheme that groups a set of atoms possibly appearing in the given structural position.
Not only can list atoms be used to merge similarly behaving but structurally distinct chemical functionalities, but they are also commonly applied to replace interchangeable atoms, like e.g. halogens. Instead of separately defining the following reactions,
|
Reaction |
Example |
|
Friedel-Crafts alkylation with alkyl chloride |
|
|
Friedel-Crafts alkylation with alkyl bromide |
|
|
Friedel-Crafts alkylation with alkyl iodide |
|
|
Friedel-Crafts alkylation with alkyl fluoride |
|
we can simply say:
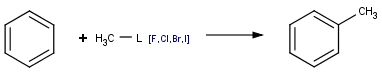
Would you like to further improve your reaction schemes? Visit Reaction Rules for Chemical Intelligence!