Match type
For exact and substructure type searches, the match type can be also set. The match of your choice can be selected by using the corresponding checkboxes.
-
2D match
The search for structures is initiated disregarding the stereochemistry (tetrahedral stereo, double bond stereo, etc.) of the query and target structures. E.g. when searching for (S)-enantiomer and checking the 2D search option, in an exact type search, the result will return also the plain structure beside the (R)-enantiomer ( Figure S4 ).
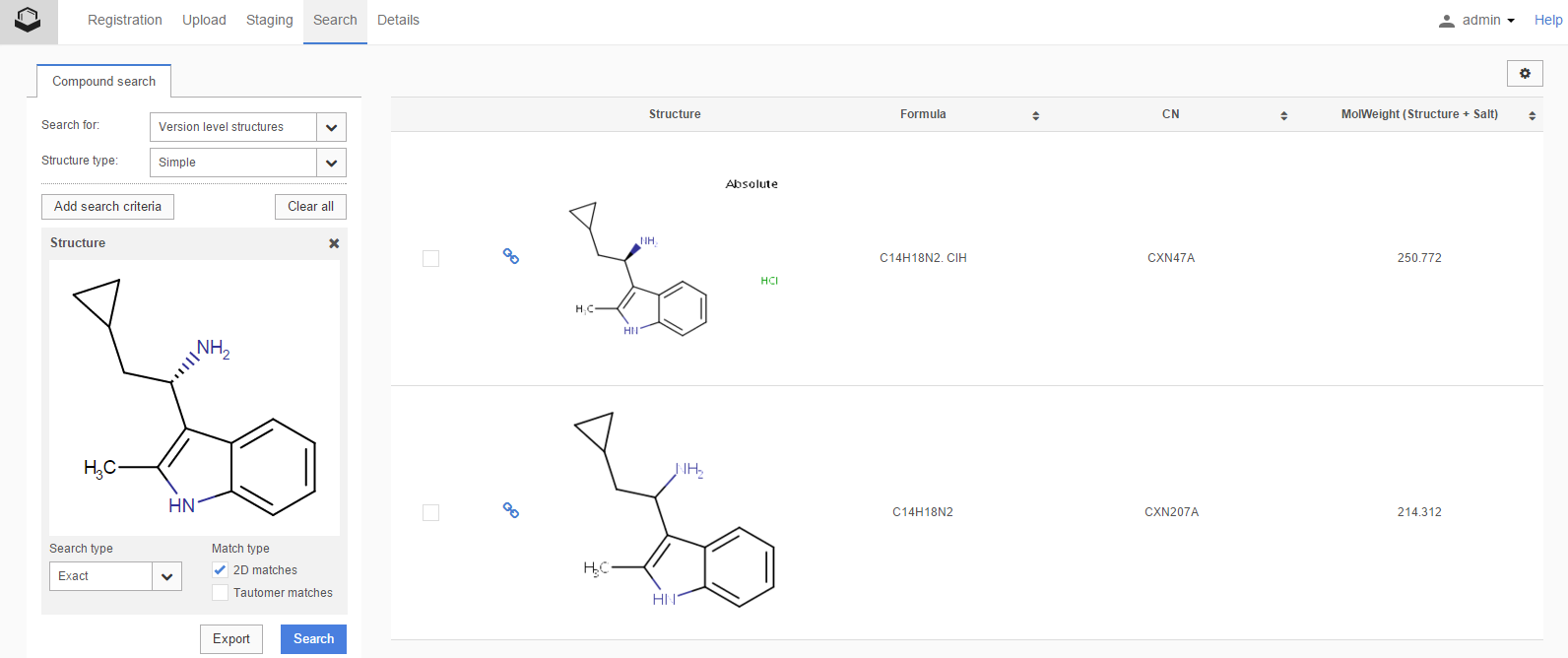
Figure S4. Different search results for 2D match Search option
Currently the matches involving CSTs (although are considered 2D matches during submission or amendment), are displayed as 'Exact' match types on the Search page because no chemical stereo information is disregarded. Therefore those hits are being returned ( Figure S5 ) also after an Exact search (when the 2D Search option is not selected).
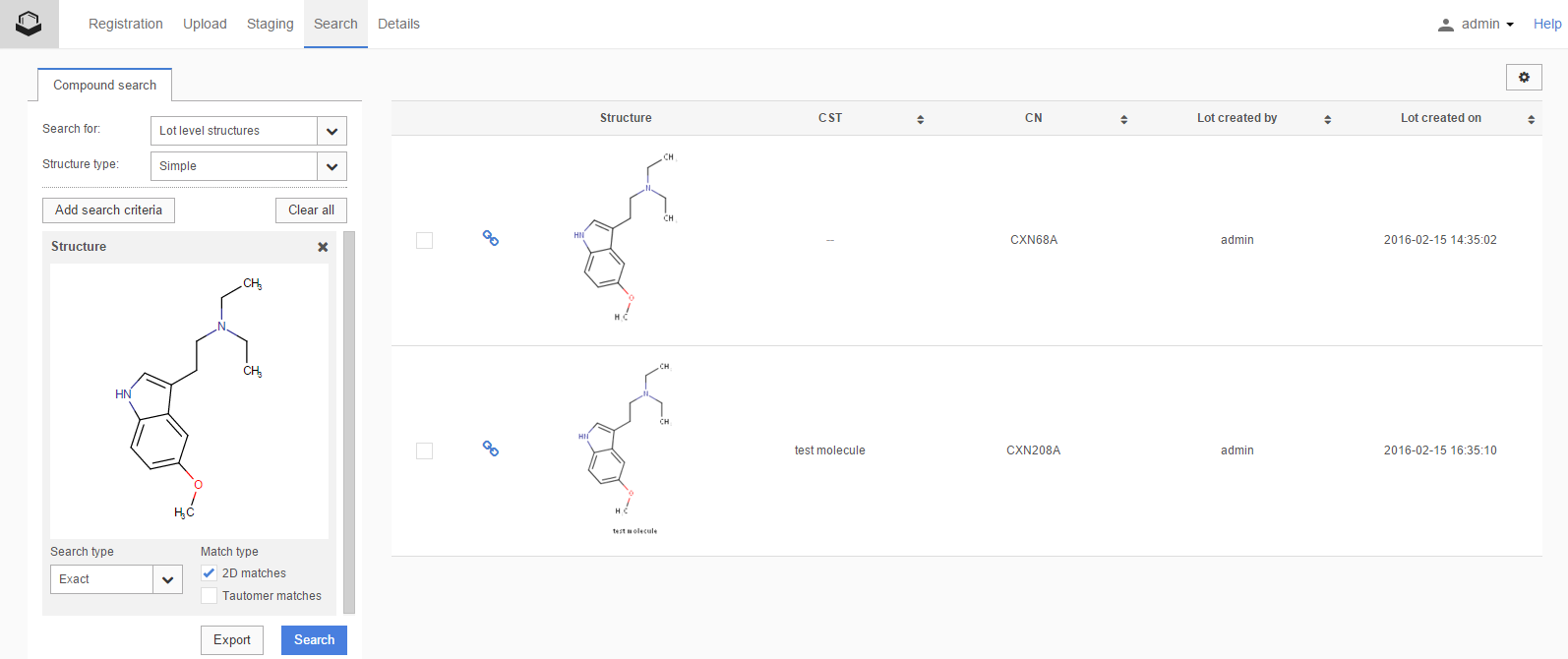
Figure S5. Exact search result for 2D match including a CST
-
Tautomer match
The search is performed by a JChem tautomer search, which includes lactim-lactam, keto-enol, imine-amine, and other common proton-shift tautomerism cases.
In the following example, a substructure search with keto-enol tautomer match is performed (Figure S6).
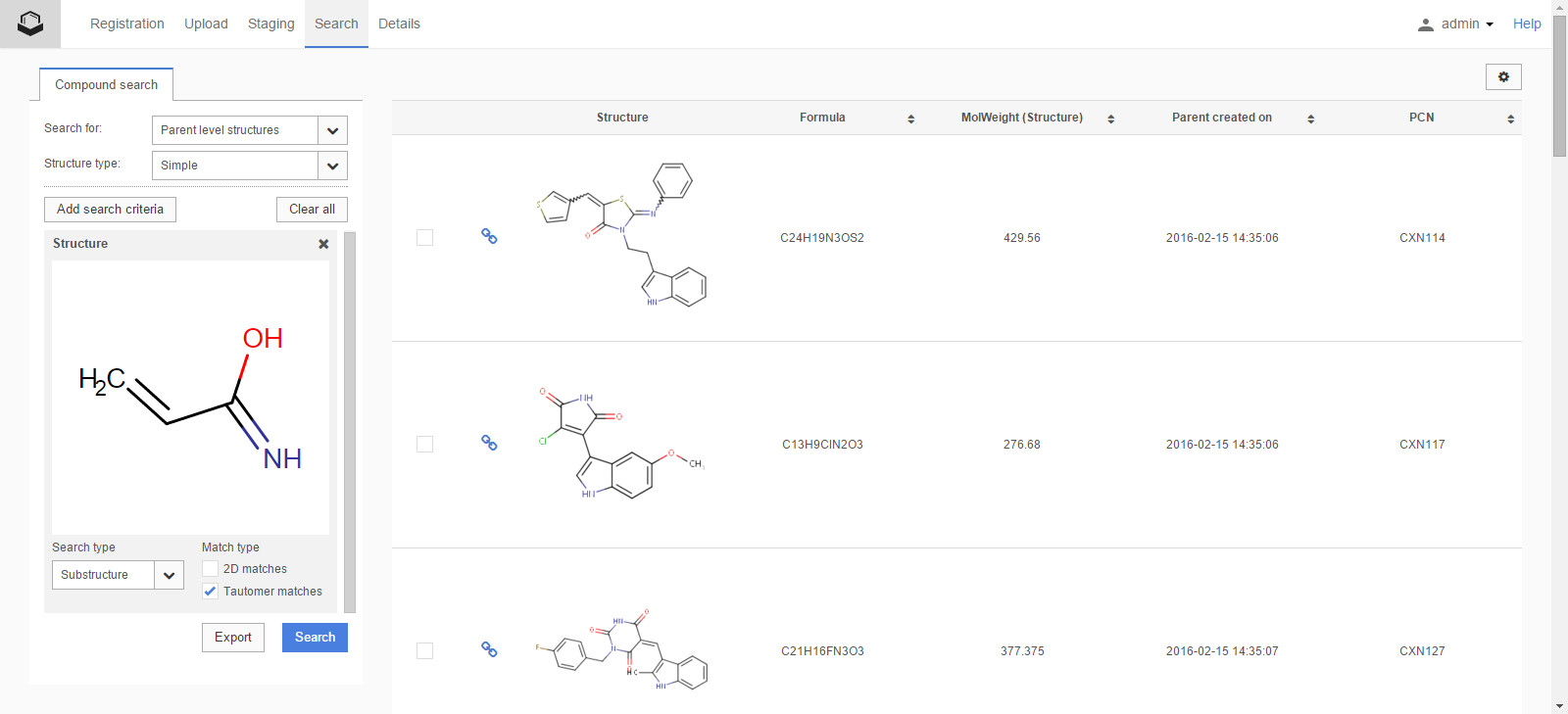
Figure S6. Substructure search result for tautomer match