Regioselectivity
The question of regioselectivity comes up when certain reaction sites of a reactant are preferred over others. Regioselective reactions show strong preference for forming particular products from a set of possible reaction outcomes.The smart reactions of Reactor generate products in line with chemists' expectations regarding regioselectivity.
Arene substitution patterns
The positions of substituents on an aromatic system in relation to each other can be referred as the arene substitution pattern. Aromatic substitution reactions often favour a given arene substitution pattern (ortho, para or meta). The smart reactions of Reactor are able to take into consideration such preferences. Thus, Reactor generates synthetically reasonable products.
Let's look at some results of the Friedel Crafts acylation reaction performed in Reactor. This reaction acylates aromatic compounds by means of acyl halides in the presence of Lewis acids. The methyl group of toluene directs newly entering groups into ortho and para positions.
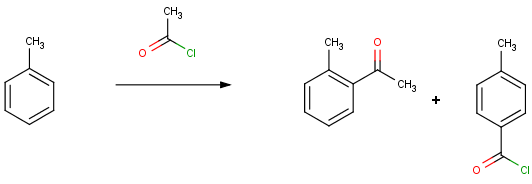
In accordance with this, toluene provides ortho and meta derivatives as main products in Reactor.
The presence of some functional groups prevents the Friedel Crafts reaction such as for example a nitro group attached to the benzene ring.
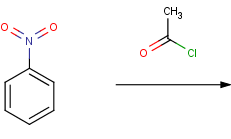
Nitrobenzene is not acylated in Reactor since the nitro group deactivates the ring.
To sum it up, applying proper settings, Reactor recognizes the most reactive sites of the reactant molecules.
Addition-elimination reactions
Reactions with addition mechnism are often guided by the Markovnikov's rule. This rule states that upon the addition of hydrogen halides to an alkene, the hydrogen becomes attached to the carbon having more hydrogen atoms (alternatively, having fewer alkyl substituents).
Reactor can obey this rule when performing virtual reaction. For example the built-in reaction Hydrogen halide addition to alkenes (Markovnikov) gives the following result for an asymmetric alkene:
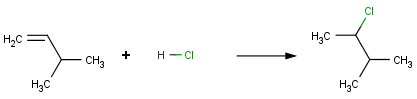
Here, hydrogen is added to the carbon atom having greater number of hydrogens as stated by the Markovnikov's rule.
Since this rule may be subject to exceptions, anti-Markovnikov reactions are also provided in the ChemAxon reaction library (e.g. Hydrogen bromide addition to alkenes (Anti-Markovnikov)).
In case of elimination reactions, Zaitsev's rule usually guides the elimination process. According to this rule, the hydrogen is removed from the carbon having the fewest hydrogen substituents.
This empirical rule can also be formulated in Reactor. For example the reaction Beta HX elimination (Zaitsev elimination) proceeds in Reactor as follows:
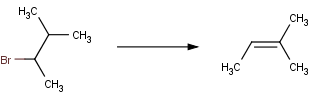
In accordance with Zaitsev's rule, hydrogen leaves the carbon atom having smaller number of hydrogens.
How to embed regioselectivity in custom reactions? Use the selectivity rules to define a smart reaction in terms of regioselectivity!