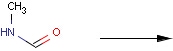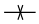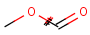Special search types: Reaction search
JChem supports the search of reactions with substructure or reaction queries. (In addition to the reaction search features described in this section, all query features of non-reaction search can be used.)
Besides reaction structural searches, reaction similarity calculation and search is also available. For more details, see the reaction similarity documentation .
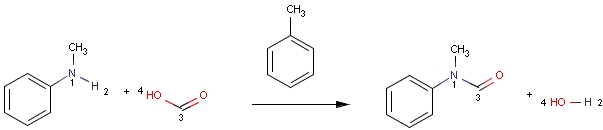
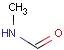
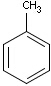
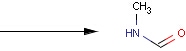
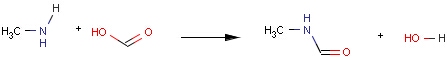
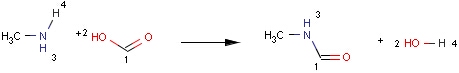
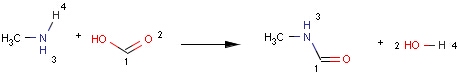
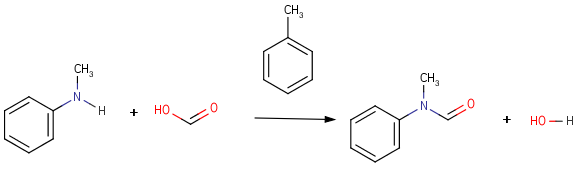
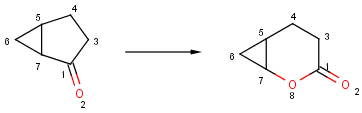
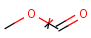
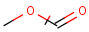
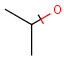
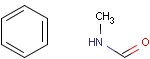
Structures to the left of the reaction arrow are reactants (starting materials), structures to the right of the reaction arrow are products, and those molecules drawn just above or below the arrow are agents (ingredients). Corresponding atoms containing changing bonds (created, destroyed or modified) are marked with map numbers both in the reactants and in the products (Table 1.).
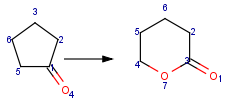
Searching for a substructure in a reaction equation does not differ from the classical substructure search process described above. Any matching in any reaction components (reactants, agents, products) is a hit. This is not the case when the query itself is a reaction.
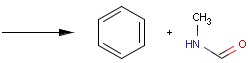
Table 1. Searching for simple query structure in reaction
|
|
target |
|
|
|
||
|
query |
|
|
|
|
|
|
|
|
|
|
Component types
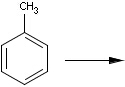
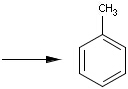
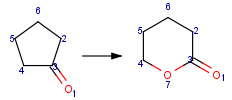
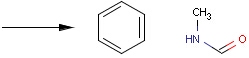
Reaction queries are not necessarily complete reactions. Reaction queries sometimes contain reactants only. In this case, the search engine retrieves reactions containing reactants matching to the given structure. When just a product is specified in the query, those reactions will be returned which contain matching products (Table 2).
Table 2. Searching for query structure in reaction components
|
|
target |
|
|
|
||
|
query |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See also SMARTS component level grouping.
Atom maps
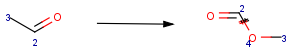
When a reaction query has mapped atoms, the reaction center of a matching reaction is mapped correspondingly. Although, the actual value of the map numbers might be different in the query and the target, the hit atoms have to be paired exactly as they are in the query (Table 3).
Table 3. Searching by mapped reaction queries
|
|
target |
|
|
|
||
|
query |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Note : If you plan to execute database searches with mapped reaction queries then the target reactions must be mapped before database import. We offer Standardizer application, built in JChem, for mapping reactions.
Reacting center bond query feature
You can restrict your reaction search by applying reacting center query features on bonds to express the bond's role in the reaction mechanism. Table 4. describes these query features.
Table 4.
|
Symbol on bond |
Name |
Description |
|
|
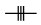
Center |
The bond takes part in the reaction. (Its bond type changes or the bond is created or disappears in the reaction.) |
|
|
Make or break |
The bond is created or disappears in the reaction. (Depending if it is present on the reactant or product side.) |
|
|
Change |
The bond remains in the reaction, but its bond type changes, for example from single to double. |
|
|
Make and change |
Currently it works exactly as "Center" above |
|
|
Not center |
The bond must not change in the reaction. |
Restrictions:
-
Our method requires atom maps to identify the above reacting center bond categories. For this reason, it is best if the target(database) reactions are fully mapped or at least atoms with changing bonds are mapped. (ChemAxon-style mapping.)
-
If the atom maps are missing, we try to automap the target(database) reactions, but this may introduce errors. Automappings are taken into account exclusively at the evaluation of reacting center bond query features.
-
If atom mapping is not unambiguous (e.g. the same atom map appears two or more times on the product side, or alternatively on the reactant side) then only one of the mapped atoms will be used to calculate the reacting center and it is arbitrary which one is used.
Table 5. shows some examples.
Table 5.
|
Query |
Target |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Note : Reacting center bond features of reactions in database are not considered.
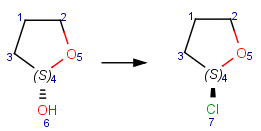
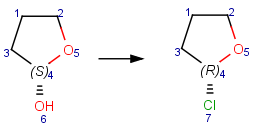
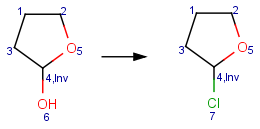
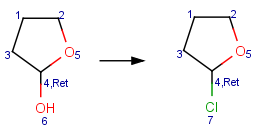
Reacting center stereo query feature
In case of mapped reactions, reacting center stereo query features - inversion and retention - can be applied on the chiral atom in the reacting center. In case of unmapped reactions, these query features are not applicable.
-
Inv: inversion of the reacting center stereo bond
-
Ret: retention of the reacting center stereo bond Table 6. displays some examples.
Table 6. Effect of reacting center stereo query features during reaction search
|
Query |
Target |
|
|
|
|
|
|
|
|
|
|
|
|
|
Component identification
A query structure occasionally consists of some disjunct fragments. Since these fragments belong to a single reaction component in the query, their corresponding hits must belong to a single component as well. Two components of a reaction query are matching to two components of a target reaction (Table 7).
Table 7. Component identification during reaction search
|
|
target |
|
|
|
||
|
query |
|
|
|
|
|
|
|
|
|
|